The Art and Design of Genetic Screens Escherichia Coli
- Methodology article
- Open Access
- Published:
Frontward genetic screen of homo transposase genomic rearrangements
BMC Genomics volume 17, Article number:548 (2016) Cite this commodity
Abstract
Background
Numerous man genes encode potentially agile Deoxyribonucleic acid transposases or recombinases, merely our understanding of their functions remains express due to shortage of methods to profile their activities on endogenous genomic substrates.
Results
To enable functional assay of human being transposase-derived genes, we combined forward chemical genetic hypoxanthine-guanine phosphoribosyltransferase i (HPRT1) screening with massively parallel paired-end DNA sequencing and structural variant genome assembly and analysis. Hither, nosotros report the HPRT1 mutational spectrum induced by the human being transposase PGBD5, including PGBD5-specific bespeak sequences (PSS) that serve every bit potential genomic rearrangement substrates.
Conclusions
The discovered PSS motifs and loftier-throughput frontward chemical genomic screening approach should show useful for the elucidation of endogenous genome remodeling activities of PGBD5 and other domesticated human being DNA transposases and recombinases.
Background
The human genome contains over 20 genes with similarity to Dna transposases [1]. In addition, transposons are a major source of structural genetic variation in human populations [2]. Recently, human THAP9 and PGBD5 have been institute to mobilize transposons in homo cells [3, 4]. This discovery raises the possibility that, similar to the RAG1 recombinase [five], these endogenous human transposases may catalyze human genome rearrangements during normal somatic jail cell development or in singled-out disease states. The man genome contains thousands of genetic elements with apparent sequence similarity to transposons, but their evolutionary divergence hinders the identification of elements that may serve as substrates for endogenous human being transposases in full general [vi], and PGBD5 in particular [4].
In classical genetics, forrard chemical genetic screens have been successfully used to identify spontaneous mutations in leaner, yeast and fly [7–x]. Such approaches use DNA sequencing of cells based on chemical resistance due to positive or negative phenotypic selection. For forwards genetics of mammalian and man cells, mutational analysis of the hypoxanthine-guanine phosphoribosyltransferase one (HPRT1) gene based on the resistance to toxic purine analogues such as 8-aza- or half dozen-thio-guanine (referred to as thioguanine) has been used; for overview, run across [xi]. Analysis of HPRT1 has several advantages for forrard genetic screens: i) HPRT1 is on the Ten chromosome and therefore functionally hemizygous, ii) HPRT1 encodes a unmarried domain globular protein in which alterations of whatsoever of its nine exons are expected to impact enzymatic activity, and iii) mutations tin be selected both positively and negatively, enabling the specific identification of distinct mutations, as opposed to general factors controlling cellular genomic stability. Indeed, HPRT1-based forward genetic screens take been successfully used to characterize chemical mutagens [12, 13]. In human lymphocytes, this assay has also been used to identify RAG1-mediated mutations of HPRT1, and to elucidate cryptic recombination signal sequences [fourteen, 15].
Here, nosotros sought to develop a forward genetic screening approach suitable for the elucidation of endogenous genomic substrates of human being Deoxyribonucleic acid transposases and recombinases. Depending on cell blazon and presence of endogenous co-factors, this assay should allow for Dna transposition and recombination, or alternatively, nuclease-mediated Deoxyribonucleic acid rearrangements facilitated by endogenous DNA sequence substrate preferences. Using negative and positive thioguanine resistance selection, combined with massively parallel DNA sequencing, we used HPRT1 screening to investigate the nuclease activity of PGBD5 on human genomic substrates.
Results
The homo HPRT1 gene contains 12 annotated DNA transposon copies (Additional file 1: Tabular array S1). These transposons are simply distantly related to piggyBac transposons that are evolutionarily related to the potential substrates of PGBD5 [4]. We hypothesized that under potent selective pressure, PGBD5 may exhibit enzymatic action on sequences in the HPRT1 gene with sufficient similarity to its endogenous substrates, be they piggyBac-related sequences or non. To test this hypothesis, we adjusted the HPRT1 mutation assay in which cells containing inactivating HPRT1 mutations can be negatively or positively selected by growth in media containing hypoxanthine-aminopterin-thymidine (HAT) or thioguanine, respectively [xvi]. To maximize the sensitivity of this assay, nosotros used male BJ fibroblasts containing a single re-create of the X-linked HPRT1 gene [17].
To clarify specific changes induced by man PGBD5, we generated isogenic cell lines by using lentiviral transduction to limited GFP-PGBD5 and control GFP, and confirmed stable and equal transgene expression using immunoblotting (Fig. 1a). To eliminate HPRT1 variants induced by spontaneous gene mutations and enable the specific choice of those induced past PGBD5, we grew cells in the presence of HAT medium for 15 doublings [18]. We confirmed that the expression of GFP-PGBD5 did not alter the intrinsic sensitivity of BJ cells to thioguanine, as assessed using clonogenic assays and analysis of the dose–response to thioguanine by these cells (Fig. 1b and c). The fractional clonogenic efficiencies of thioguanine resistance induction were 0.027 and 0.028 for cells expressing GFP and GFP-PGBD5, respectively, estimated from the number of thioguanine-resistant colonies, consistent with the lack of general mutagenic action by PGBD5 [12, thirteen]. To generate cells with de novo HPRT1 mutations, nosotros grew cells expressing GFP-PGBD5 or GFP control in the presence of thioguanine for 10 jail cell divisions, respective to approximately 1 month in civilization. To ostend the generation of clones with inactivating HPRT1 mutations, we determined the ability of thioguanine-selected cells to grow in the Hat medium. We observed more than 3.5-fold decrease in the number of viable cells upon Chapeau handling, indicating that the majority of the cells had acquired resistance to thioguanine (Fig. 1d). Consistent with the inactivation of HPRT1 in these cells, thioguanine-selected cells exhibited increased resistance to acute handling with thioguanine equally compared to unselected control cells (Fig. 1e). In agreement with this notion, we observed no detectable HPRT1 protein expression by immunoblotting (Fig. 1f), suggesting that the majority of thioguanine-resistant cells inactivated HPRT1 due to mutations that led to the loss of HPRT1 protein expression.
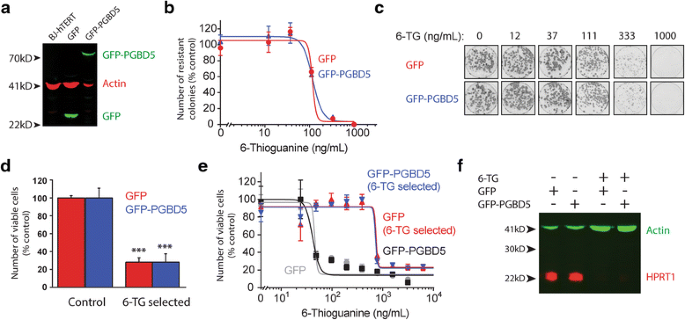
Induction of thioguanine resistance and loss of HPRT1 expression in isogenic cells expressing DNA transposase PGBD5. a Stable expression of GFP-PGBD5 and control GFP in BJ-hTERT cells, equally assessed by Western blotting against GFP; β-actin serves as loading control. b Clonogenic efficiency of BJ-hTERT cells stably expressing GFP (red) and GFP-PGBD5 (bluish) equally a function of varying thioguanine concentrations upon thioguanine resistance option. c Representative photographs of resistant colonies stained with Crystal Violet. d Thioguanine choice of both GFP and GFP-PGBD5 expressing cells yields cells that lack HPRT1 action, as assessed by hypoxanthine-aminopterin-thymidine (Hat) handling; *** denotes p = 2.two × 10−5 and 9.6 × 10−4 for the comparisons betwixt control and thioguanine selected GFP and GFP-PGBD5, respectively. d Thioguanine selection yields thioguanine-resistant cells, as assessed by cellular ATP luminescence assay of GFP (cherry-red) and GFP-PGBD5 (bluish) expressing cells, every bit compared to control cells (gray and black). due east Western blot for HPRT1 in BJ-hTERT cells expressing GFP and GFP-PGBD5 upon thioguanine selection; β-actin serves as loading control. All error confined represent standard deviations of 3 biological replicates
Prior structural studies of HPRT1 gene mutations take used Southern blotting or cDNA sequencing [19], limiting the detection and sequence analysis of structural variants that involve rearrangements of introns and other non-exonic sequences. To overcome these limitations and enable comprehensive and sensitive sequence assay of rearrangements involving potential transposon sequences in HPRT1, nosotros designed a specific panel of polymerase concatenation reaction (PCR) amplicons spanning the unabridged 43 Kb sequence of the man HPRT1 factor, including introns, exons, and v′ and 3′ untranslated regions (Fig. 2a and Additional file 2: Table S2). Massively parallel paired-end Dna sequencing of the resultant amplicons, producing millions of sequence reads spanning the HPRT1 locus, should enable the recovery of rare Deoxyribonucleic acid mutations without the need for single cell cloning that is limited by clonal fitness, equally observed for RAG1-induced HPRT1 mutations in lymphocytes [20, 21].
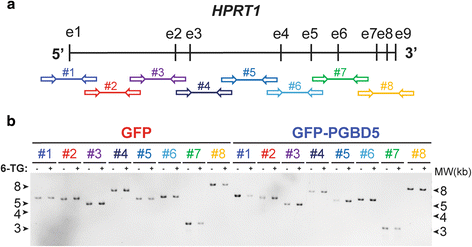
Massively parallel Deoxyribonucleic acid sequencing combined with PCR amplification for loftier-resolution mutational analysis of HPRT1. a Schematic of the human being HPRT1 gene structure with vertical bars and horizontal arrows denoting exons and amplicons equally numbered. b Photograph of ethidium bromide-stained and electrophoretically-resolved HPRT1 PCR amplicons of genomic Deoxyribonucleic acid isolated from GFP (red) and GFP-PGBD5 (blueish) expressing cells before (−) and afterward (+) thioguanine resistance selection
To exam this prediction, we isolated genomic DNA from thioguanine-resistant cells expressing GFP-PGBD5 or GFP, and amplified their HPRT1 loci using long-range PCR (Fig. 2a). Consistent with prior observations of HPRT1 mutations that were either subclonal or involved variants non resolvable by electrophoresis [14, fifteen, 21], resultant amplicons exhibited no apparent differences in electrophoretic gel mobility between thioguanine-resistant and command cells in the presence or absence of PGBD5 (Fig. 2b). To facilitate the recovery of polyclonal populations of HPRT1 mutants, we used massively parallel paired-end Illumina Dna sequencing of resultant genomic amplicons to generate more than than 32,000 sequence reads at 99 % of nucleotide bases. These data take been deposited to the Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra/, accretion number SRP068848), with the candy and annotated data bachelor from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.t748p).
To enable comprehensive assay of PGBD5-induced HPRT1 mutations, we combined two recently adult algorithms CRISPResso and laSV that permit the identification and analysis of both small and large structural variants in ultra-high coverage Deoxyribonucleic acid sequencing data at base-pair resolution [22, 23]. We observed that both GFP and GFP-PGBD5 expressing cells acquired unmarried nucleotide and small indel mutations of HPRT1 (Fig. 3a). Consistent with the functional loss of HPRT1 expression (Fig. 1f), we observed a relative excess of exonic equally compared to intronic variants in thioguanine-resistant cells (Fig. 3a, right). There was no meaning difference in the frequencies of single nucleotide and pocket-size indel mutations of HPRT1 between GFP and GFP-PGBD5 expressing cells (Fig. 3a, left). Although both GFP-PGBD5 and GFP expressing cells adult thioguanine resistance at least in part due to the acquisition of inactivating single nucleotide and modest indel mutations, this finding suggests that PGBD5 activity on human genomic substrates does not directly generate such mutations at the efficiency required for thioguanine resistance selection.
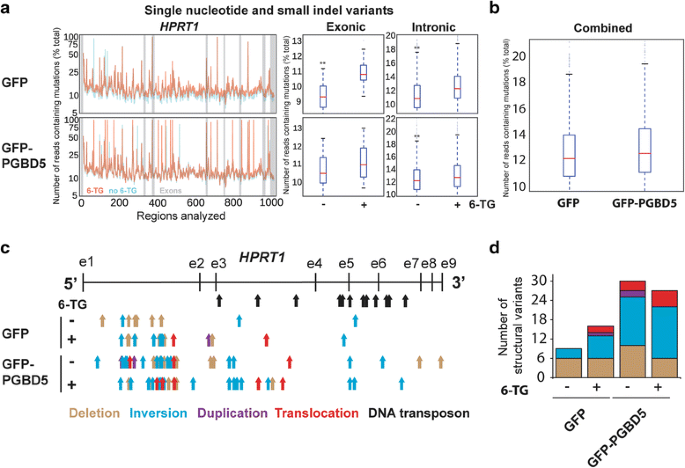
Comprehensive genomic assay of HPRT1 mutations reveals PGBD5-mediated induction of complex genomic rearrangements. a (left) Distribution of the mutational frequency (y-axis) and the location (x-axis) of single nucleotide variants (SNV) and small indels in HPRT1 of cells before (blue) and after (orange) thioguanine resistance option. Exons are denoted by gray bars. (right) Comparative assay of the frequencies of SNVs and indels in HPRT1 earlier (−) and after (+) thioguanine resistance option reveals no meaning differences betwixt GFP and GFP-PGBD5 expressing cells; * and ** denote p < 0.05 and p < 0.01 for exonic and intronic variants, respectively (Exonic GFP p = 8.32e-vi, exonic GFP-PGBD5 p = 0.09, intronic GFP p = 2.77e-36, intronic GFP-PGBD5 p = 4.77e-06). b Combined comparative analysis of the frequencies of SNVs and indels in HPRT1 in GFP and GFP-PGBD5 expressing cells (p = 7.90e-four). c Distribution of the locations of the 5′ ends of complex structural variants in cells earlier (−) and after (+) thioguanine resistance choice, as detected by laSV and marked by arrows denoting deletions (brown), inversions (blue), duplications (purple), and translocations (cerise). Black arrows mark annotated DNA transposons. d Expression of GFP-PGBD5 leads to induction of complex structural variants before (−) and after (+) thioguanine resistance selection (Full number of SVs GFP vs. PGBD5, p = 0.001, Poisson exam, 95 % conviction interval 0.262–0.713)
On the other hand, nosotros observed that cells expressing GFP-PGBD5 had an excess of complex structural variants as compared to GFP control cells (Fig. 3c, d and Table 1). Specifically, we establish that PGBD5-expressing cells contained significantly greater numbers of inversions in HPRT1 as compared to control GFP cells (p = 0.001, Poisson test with an exact reference distribution, 95 % confidence interval 0.14–0.68) and too independent significantly greater total numbers of complex structural variants (p < 0.001, Poisson test with an exact reference distribution, 95 % conviction interval 0.26–0.71) (Fig. 3c). Furthermore nosotros observed that some rearrangements occurred in the absence of thioguanine option. This is consequent with the preserved nuclease activity of PGBD5 which may induce such structural rearrangements in human genomes by virtue of DNA double strand breaks alone, like to the RAG1 recombinase [14, 15]. PGBD5-induced genomic rearrangements in HPRT1 did not appear to involve annotated human transposable elements (Fig. 3c), consequent with the absenteeism of canonical piggyBac-derived transposons within the human being HPRT1 gene [4].
Previously, we institute that the genomic integration of transposons by PGBD5 required specific DNA substrate sequences containing inverted final repeats with GGG terminal motifs [4]. We reasoned that potential human endogenous PGBD5 substrates in HPRT1 may be identified by the presence of inverted concluding repeats at the sequences flanking the breakpoints of the observed structural variants (specific definition described in the Methods). Using this approach, we identified 13 final elements flanking the structural variant breakpoints in the PGBD5-expressing but not in the control GFP cells (Fig. 4 and Table two). The identified structural variant breakpoint sequences exhibited specific motifs in the PGBD5 but non in the command GFP cells (Fig. 4), as assessed using sequence entropy analysis and implemented in the MEME algorithm [24, 25]. Consequent with their thioguanine resistance selection, 2 out of 13 structural variants involving these sequence motifs were predicted to crusade inactivation of HPRT1 past causing exonic deletions, similar to prior studies of RAG1-induced HPRT1 inactivation [12]. We confirmed that the identified PGBD5-specific sequence motifs occur in the HPRT1 factor at approximately the same frequency every bit the rest of the human genome, and therefore are not enriched in our assays just considering of their genomic distribution (p = 0.066, binomial-test). While they differ in sequence from the canonical piggyBac-derived inverted terminal repeats, PGBD5-specific sequence motifs in HPRT1 are enriched for concluding GGG nucleotides and lack thymines (Fig. four), in understanding with the structural requirements observed in previous studies using synthetic transposon substrates [iv]. While these sequences motifs may establish specific PGBD5 signal sequences (PSS) that are associated with PGBD5-induced genomic rearrangements, it remains to be determined whether PGBD5 induces a similar variety of genomic rearrangements equally part of its endogenous activities, or alternatively, whether it mediates true Dna transposition with excision and insertion of specific PSS-containing inverted terminal echo mobile elements.
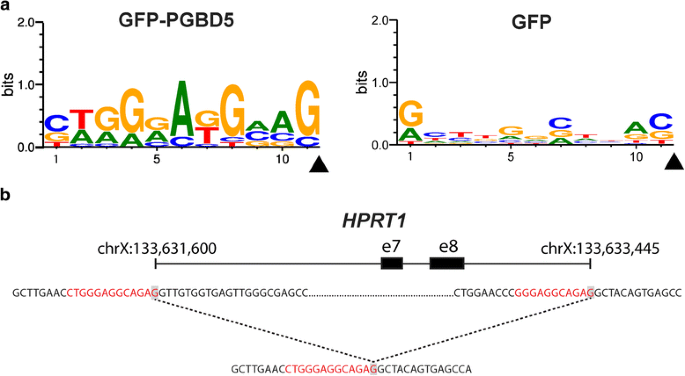
PGBD5-associated HPRT1 structural variants comprise terminal betoken sequences. a Analysis of the sequences flanking structural variant breakpoints demonstrating clan of specific betoken sequence motifs in cells expressing GFP-PGBD5 (top), simply not GFP control (lesser). X-centrality denotes nucleotide sequence logo position, and y-axis denotes information content in bits. Black arrowheads mark the location of the breakpoints. b Breakpoint sequences of a representative structural variant (deletion bnd_11) showing PSS sequence at both breakpoints of a deletion
Discussion
In all, our findings indicate that human PGBD5 can induce structural variation and genomic rearrangements of endogenous man HPRT1 loci. The identification of potential PGBD5 signal sequences in human genomes using the HPRT1 forwards genetic screen represents a crucial get-go step in defining its endogenous genomic substrates in vertebrates and humans. Consistent with the singled-out evolutionary history and developmental neuronal expression of PGBD5, identified PSS motifs are distinct from the recombination betoken sequences (RSS) described for RAG1 in lymphocytes [14, fifteen]. Importantly, identified PSS motifs exhibit only limited similarity to approved piggyBac transposons, namely preference for terminal GGG nucleotides, in back up of the singled-out phylogeny of PGBD5 as compared to other piggyBac-derived genes in vertebrates [4]. Since our analysis was limited to genomic rearrangements of human HPRT1 in BJ fibroblasts the presence of thioguanine selection, it is possible that PGBD5 may exhibit different sequence preferences and remodeling activities in neurons and diseased cells where it is endogenously expressed. Our analysis did not identify bona fide 'cut-and-paste' DNA transposition in HPRT1, and it remains to be determined whether PGBD5 catalyzes DNA transposition of endogenous human mobile elements, or simply their nuclease-mediated DNA rearrangements. The described PSS motifs now provide essential templates for future functional studies of PGBD5-induced genomic remodeling.
Conclusions
Recent discovery of active human THAP9 and PGBD5 DNA transposases, combined with the functional recombination activity of RAG1, suggests that other endogenous transposase-derived genes may catalyze as of all the same unknown cell-specific somatic or germ-line rearrangements in vertebrates and humans. While their identification has been substantially empowered by whole-genome sequencing, conclusion of their functional activities has been hindered by the lack of knowledge of their endogenous substrate sequences. We expect that the integration of forward genetic screening with massively parallel Deoxyribonucleic acid sequencing, every bit we accept done here, and structural variant genome analysis using methods such as CRISPResso/laSV should permit the determination of the genome remodeling activities of endogenous besides as engineered genome editing enzymes. While leveraging the advantages of negative and positive selection of HPRT1 forward genetic screening for specificity, this approach additionally benefits from improved sensitivity, enabling the identification of both elementary and complex structural variants at base-pair resolution. This is express only by sequencing coverage, without the need for single cell cloning that may be compromised by cell fitness effects. Finally, nosotros anticipate that the reported PGBD5 signal sequences will lead to the elucidation of its function in health and disease.
Methods
Reagents
All reagents were obtained from Sigma-Aldrich, unless otherwise specified. Synthetic oligonucleotides were synthesized and purified by High Performance Liquid Chromatography (HPLC) by Eurofins MWG Operon (Huntsville, AL, Us).
Jail cell culture
BJ-hTERT cells were obtained from the American Type Civilisation Collection (ATCC, Manassas, Virginia, USA). The identity of all cell lines was verified by Curt tandem repeat analysis (STR) assay and lack of Mycoplasma contamination was confirmed past Genetica Deoxyribonucleic acid Laboratories (Burlington, NC, United states). Prison cell lines were cultured in Dulbecco'southward Modified Eagle Medium (DMEM) supplemented with ten % fetal bovine serum and 100 U / ml penicillin and 100 μg / ml streptomycin in a humidified atmosphere at 37 °C and five % COtwo.
Plasmid constructs
Human PGBD5 cDNA (Refseq ID: NM_024554.3) was cloned as a GFP fusion into the lentiviral vector pReceiver-Lv103-E3156 (GeneCopoeia, Rockville, Medico, USA). Lentivirus packaging vectors psPAX2 and pMD2.Yard were obtained from Addgene [26]. Plasmids were verified by brake endonuclease mapping and Sanger sequencing, and deposited in Addgene (https://www.addgene.org/Alex_Kentsis/).
Lentivirus product and transduction
Lentivirus production was carried out as described previously [27]. Briefly, HEK293T cells were transfected using TransIT with 2:1:1 ratio of the pRecLV103 lentiviral vector, and psPAX2 and pMD2.G packaging plasmids, according to manufacturer's instructions (TransIT-LT1, Mirus, Madison, WI). Virus supernatant was collected at 48 and 72 h mail service-transfection, pooled, filtered and stored at −80 °C. BJ-hTERT cells were transduced with virus particles at a multiplicity of infection of v in the presence of viii μg/ml hexadimethrine bromide. Transduced cells were selected for 2 days with puromycin (5 μg/ml).
Western blotting
To analyze protein expression by Western immunoblotting, i million transduced cells were suspended in 340 μl of lysis buffer (4 % sodium dodecyl sulfate, vii % glycerol, i.25 % beta-mercaptoethanol, 0.two mg/ml Bromophenol Blue, 30 mM Tris–HCl, pH vi.viii). Lysates were cleared by centrifugation at 16,000g for 10 min at 4 °C. Antiseptic lysates (thirty μl) were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electroeluted using the Immobilon FL PVDF membranes (Millipore, Billerica, MA, Us). Membranes were blocked using the Odyssey Blocking buffer (Li-Cor), and blotted using antibodies against GFP (mouse anti-human, 1:500, clone 4B10, Cell Signaling Engineering, Beverly, MA), β-actin (rabbit anti-human being, 1:5000, clone 13E5, Cell Signaling Applied science, Beverly, MA), HPRT1 (rabbit anti-human, 1:1000, clone ab10479, Abcam, Cambridge, MA) and β-actin (mouse anti-human, ane:5000, clone 8H10D10, Cell Signaling Engineering science, Beverly, MA). Blotted membranes were visualized using goat secondary antibodies conjugated to IRDye 800CW or IRDye 680RD and the Odyssey CLx fluorescence scanner, according to manufacturer'south instructions (Li-Cor, Lincoln, Nebraska).
Hypoxanthine-aminopterin-thymidine (HAT) medium selection
Chapeau medium prepared using the l× Lid supplement (Thermo Fisher Scientific) and DMEM medium with 10 % fetal bovine serum and 100 U / ml penicillin and 100 μg / ml streptomycin. Media was replaced twice weekly and cells were grown in the presence of Lid selection for fifteen doublings, respective to approximately 5 weeks.
Thioguanine selection
Cells were cultured in the presence of 120 ng/ml of six-thioguanine for x doublings, corresponding to approximately four weeks. Media was replaced twice weekly.
Prison cell viability and colony formation assays
For cell viability assays, cells were seeded at a density of 200,000 cells per well in 6-well plates (Corning Life Sciences, Corning, NY, USA). Xx four hours after seeding, medium was replaced with HAT medium. The number of viable cells was counted 2 days after treatment using Trypan Blue staining using the Neubauer hematocytometer co-ordinate to the manufacturer's instructions (Thermo Fisher Scientific).
For clonogenic assays, cells were seeded at a density of 10,000 cells per 10-cm dish and treated with 6-thioguanine (0–1 μg/ml) for two weeks. Resultant colonies were fixed with methanol, stained with Crystal Violet, and counted manually using a spatial grid.
To appraise cell viability of cells later handling with 6-thioguanine, cells were seeded into 9-well plates (Thermo Fisher Scientific, Waltham, MA, USA) at a density of 1000 cells per well. Cell were treated with half dozen-thioguanine (0–1 μg/ml) 24 h after seeding. Cell viability was quantified using the CellTiter-Glo ATP content luminescence based analysis, co-ordinate to manufacturer's instructions (Promega, Madison, WI, USA).
Generation of HPRT1 amplicons
Genomic Dna was extracted from 10 million cells using the PureLink Genomic DNA Mini Kit according to the manufacturer'southward instructions (Thermo Fisher Scientific). To exponentially amplify the HPRT1 gene, we designed primer pairs every iii–8 Kb (Boosted file 2: Table S2). Amplicons were generated using 50 ng of gDNA in 50 μl reaction volumes containing 0.v μM of primers. Loci ii to viii where amplified using the Phusion Green High-Fidelity DNA Polymerase (Thermo Scientific, Waltham, MA, United states of america) with the following parameters: 98 °C for 30 s, followed by thirty cycles of 98 °C for 10 s, 65 °C for 30 southward and 72 °C for 3.5 min, and a final extension of 72 °C for 3.v min. Locus 1 was amplified using the KAPA Long Range HotStart Dna Polymerase (KAPA Biosystems, Wilmington, MA, The states) with the following parameters: 94 °C for 3 min, followed past 40 cycles of 94 °C for 25 due south, threescore °C for 15 s and 72 °C for vii min, and a terminal extension of 72 °C for vii min. PCR products were purified using the PureLink PCR purification kit according to the manufacturer'south instructions (Invitrogen Corp., Carlsbad, CA, U.s.a.).
Illumina library preparation and sequencing
Equimolar amounts of purified PCR amplicons were pooled, equally measured using fluorometry with the Qubit instrument (Invitrogen Carlsbad, CA) and sized using the BioAnalyzer 2100 instrument (Agilent Technologies, Santa Clara, CA). The sequencing library construction was performed using the KAPA Hyper Prep Kit (KAPA Biosystems, Wilmington, MA) and 12 indexed Illumina adaptors obtained from IDT (Coralville, IO), according to the manufacturer'south instructions. Subsequently quantification and sizing, libraries were pooled for sequencing on a MiSeq (pooled library input at 10 pM) using a 300/300 paired-end run (Illumina, San Diego, CA). A total of 728,000–928,000 paired reads were generated per sample. The duplication rate varied between 0.22 and 0.27 %. The data reported in this manuscript have been deposited to the Sequence Read Archive (SRA) (http://world wide web.ncbi.nlm.nih.gov/sra/, accretion number SRP068848), and the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.t748p).
Mutational and structural variant analysis
For the analysis of single nucleotide and small indel mutations, we used the CRISPResso WGS utility from the CRISPResso software using default parameters [22]. The analysis was performed on non-overlapping windows of 40 bp spanning the unabridged HPRT1 gene trunk. For the analysis of large structural variants, we used laSV with the following parameters: -s 30 -yard 63 -p 30 [23].
PGBD5 signal sequence analysis
Clustal Omega with default parameters was used for multiple sequence alignment [24]. PGBD5 bespeak sequences were defined using the following criteria: i) sequences flanking 5′ and iii′ breakpoints demonstrated at least 50 % identity less than 4 bp from the breakpoints when aligned to each other in inverted orientation, 2) aligned sequences contained no single or tandem repeats longer than v bp, and iii) no such alignments were identified at breakpoints of variants constitute in GFP control expressing cells. Sequence motifs were identified using MEME with default parameters by referencing alignments in the v′ to 3′ management with the breakpoint at the 3′ terminus [28].
Statistical analysis
Mutational frequencies were calculated equally described previously [xviii], according to the post-obit formula: Mutational frequency = −Ln(10 South / N Southward ) / −Ln(X 0 / N 0 ), where N is the number of cells seeded and X is the number of colonies formed with (S) and without (0) thioguanine option. The difference between the number of mutations across samples were compared using a Poisson test with an verbal reference distribution.
Abbreviations
6-TG, 6-thioguanine; DMEM, Dulbecco'due south Modified Eagle Medium; Lid, hypoxanthine-aminopterin-thymidine; HPLC, high functioning liquid chromatography; PCR, polymerase chain reaction; PSS, PGBD5-specific signal sequences; RSS, recombination signal sequences; SNV, single nucleotide variant; STR, Short tandem repeat analysis; SV, structural variant.
References
-
Smit AF. Interspersed repeats and other mementos of transposable elements in mammalian genomes. Curr Opin Genet Dev. 1999;nine(6):657–63.
-
Stewart C, et al. A comprehensive map of mobile chemical element insertion polymorphisms in humans. PLoS Genet. 2011;7(viii):e1002236.
-
Majumdar S, Singh A, Rio DC. The human being THAP9 factor encodes an agile P-element DNA transposase. Science. 2013;339(6118):446–8.
-
Henssen AG, et al. Genomic Deoxyribonucleic acid transposition induced past human being PGBD5. eLife. 2015;four:e10565.
-
Hiom K, Melek M, Gellert One thousand. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell. 1998;94(4):463–70.
-
Feschotte C, Pritham EJ. Dna transposons and the evolution of eukaryotic genomes. Annu Rev Genet. 2007;41:331–68.
-
Grimm S. The art and design of genetic screens: mammalian culture cells. Nat Rev Genet. 2004;5(3):179–89.
-
Shuman HA, Silhavy TJ. The art and design of genetic screens: Escherichia coli. Nat Rev Genet. 2003;iv(vi):419–31.
-
Forsburg SL. The fine art and design of genetic screens: yeast. Nat Rev Genet. 2001;ii(9):659–68.
-
St Johnston D. The art and design of genetic screens: Drosophila melanogaster. Nat Rev Genet. 2002;3(3):176–88.
-
Albertini RJ. HPRT mutations in humans: biomarkers for mechanistic studies. Mutat Res. 2001;489(one):i–16.
-
Finette BA. Assay of mutagenic V(D)J recombinase mediated mutations at the HPRT locus as an in vivo model for studying rearrangements with leukemogenic potential in children. DNA Repair. 2006;5(ix–10):1049–64.
-
Snee RD, Irr JD. Design of a statistical method for the analysis of mutagenesis at the hypoxanthine-guanine phosphoribosyl transferase locus of cultured Chinese hamster ovary cells. Mutat Res. 1981;85(2):77–93.
-
Fuscoe JC, et al. V(D)J recombinase-mediated deletion of the hprt cistron in T-lymphocytes from adult humans. Mutat Res. 1992;283(1):13–20.
-
Fuscoe JC, et al. V(D)J recombinase-like activity mediates hprt cistron deletion in human being fetal T-lymphocytes. Cancer Res. 1991;51(21):6001–v.
-
O'Neill JP, Hsie AW. Phenotypic expression fourth dimension of mutagen-induced half dozen-thioguanine resistance in Chinese hamster ovary cells (CHO/HGPRT system). Mutat Res. 1979;59(one):109–xviii.
-
Morales CP, et al. Absenteeism of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet. 1999;21(1):115–8.
-
Johnson GE. Mammalian cell HPRT gene mutation assay: test methods. Methods in Molecular Biology, Genetic Toxicology: Principles and Methods, (Springer Science+Business Media), 2012;817:55–67
-
Chen T, Harrington-Brock K, Moore MM. Mutant frequency and mutational spectra in the Tk and Hprt genes of Due north-ethyl-N-nitrosourea-treated mouse lymphoma cellsdagger. Environ Mol Mutagen. 2002;39(iv):296–305.
-
Aplan PD, et al. Disruption of the human being SCL locus by "illegitimate" V-(D)-J recombinase action. Scientific discipline. 1990;250(4986):1426–nine.
-
Albertini RJ, Nicklas JA, Skopek TR, Recio L, O'Neill JP. Genetic instability in human T-lymphocytes. Mutat Res. 1998;400(1–2):381–9.
-
Pinello L, et al. CRISPResso: sequencing analysis toolbox for CRISPR-Cas9 genome editing. Nat Biotechnol. 2015; In printing
-
Zhuang J, Weng Z. Local sequence assembly reveals a high-resolution profile of somatic structural variations in 97 cancer genomes. Nucleic Acids Res. 2015;43(17):8146–56.
-
Sievers F, Higgins DG. Clustal omega. Current protocols in bioinformatics / editoral board, Andreas D. Baxevanis … [et al.]. 2014;48:three 13 xi-13 13 16
-
Tanaka E, Bailey TL, Keich U. Improving MEME via a two-tiered significance analysis. Bioinformatics. 2014;30(fourteen):1965–73.
-
Cudre-Mauroux C, et al. Lentivector-mediated transfer of Bmi-1 and telomerase in muscle satellite cells yields a duchenne myoblast cell line with long-term genotypic and phenotypic stability. Hum Gene Ther. 2003;xiv(16):1525–33.
-
Kentsis A, et al. Autocrine activation of the MET receptor tyrosine kinase in acute myeloid leukemia. Nat Med. 2012;xviii(seven):1118–22.
-
Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings / … International Conference on Intelligent Systems for Molecular Biology; ISMB International Conference on Intelligent Systems for Molecular Biology. 1994;2:28–36.
Acknowledgments
We give thanks David Pellman for suggesting forwards chemical genetic screening for transposon discovery and Cedric Feschotte for helpful comments on the manuscript.
Funding
This work was supported by the University of Essen Pediatric Oncology Research Program (A.H.), NIH K08 DK093705 (D.E.B.), NIH K08 CA160660, Burroughs Wellcome Fund, Josie Robertson Investigator Program, and the Sarcoma Foundation of America (A.K.). Fifty.P. is supported by NHGRI Career Development Award K99 HG008399. We give thanks the MSKCC Integrated Genomics Cadre Facility and Bioinformatics Core Facility for help with DNA sequencing and analysis (NIH P30 CA008748).
Availability of data and material
Data degradation: The data reported in this manuscript accept been deposited to the Sequence Read Archive (SRA) (http://www.ncbi.nlm.nih.gov/sra/, accession number SRP068848), the Damsel Digital Repository (http://dx.doi.org/x.5061/dryad.t748p).
Authors' contributions
AH and AK designed the research, AH, EJ, CV performed the enquiry, AH, LP, JZ, ZW, DB, AK analyzed data, AH and AK wrote the manuscript in consultation with all authors. All authors read and approved the terminal manuscript.
Competing interests
The authors declare that they have no competing interests.
Ideals approval and consent to participate
Ethics approval was not required for whatever aspect of this report.
Author information
Affiliations
Corresponding author
Boosted files
Rights and permissions
Open up Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/iv.0/), which permits unrestricted utilize, distribution, and reproduction in any medium, provided y'all give appropriate credit to the original author(south) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
Well-nigh this article
Cite this article
Henssen, A.G., Jiang, Due east., Zhuang, J. et al. Forwards genetic screen of human transposase genomic rearrangements. BMC Genomics 17, 548 (2016). https://doi.org/10.1186/s12864-016-2877-x
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/x.1186/s12864-016-2877-x
Keywords
- Inverted Terminal Repeat
- Thioguanine
- HPRT1 Factor
- Recombination Bespeak Sequence
- Brusque Tandem Echo Analysis
Source: https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-016-2877-x
0 Response to "The Art and Design of Genetic Screens Escherichia Coli"
Post a Comment